Nuclear Reactions
In the chemical reactions associated with combustion, the atoms in the molecules of the active materials rearrange themselves into new, more stable, molecules in which they are more tightly bound and in the process, releasing surplus energy in the form of heat.
In nuclear reactions it is the sub-atomic particles in the atomic nucleus, the protons and neutrons, which rearrange themselves to form new elements or isotopes with more stable nuclei. In this case the energy released by the reaction in the form of kinetic energy (manifest as heat) and electromagnetic energy (gamma radiation) is millions of times greater. See Energy Content
Note: The reactions discussed on this page are all nuclear reactions not chemical reactions.
Practical applications of the use of nuclear energy to generate electricity are given on the Nuclear Energy - The Practice page
Atomic Structure
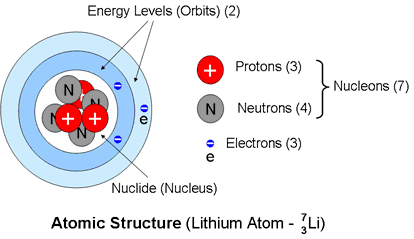
Definitions
The diagram above shows a representation of the constituents of an atom using Lithium as an example.
- Notation
- Atomic Number Z - is the number of protons in the nucleus
- Atomic Mass A - is the number of nucleons in the nucleus.
- Atomic Structure
The Lithium atom has three electrons occupying 2 energy levels and three protons giving it an atomic number Z = 3 .The nucleus also includes four neutrons making up its seven nucleons and thus a mass number A = 7The structure may be indicated by appending the mass number A after the name of the element or by indicating it as a superscript preceding the chemical symbol. Thus Lithium-7 or 7Li.The symbol may also indicate the full atomic structure ( ALiZ) by adding a subscript representing the atomic number Z preferably before, but alternatively after, the chemical symbol (depending on the capability of your word processor). Thus 7Li3
- Electron (e-) - a fundamental subatomic particle which carries a negative electric charge.It has a mass of 9.1 X 10-31 Kg, and an electric charge of 1.602 X 10-19 Coulombs (denoted as -e)
The electron gives rise to the electromagnetic properties of the atom as well as the chemical bonding with the nuclei of adjacent atoms. It can be bound to the atom or it can lead an independent life outside. - Energy Levels - Electrons can only contain distinct quanta of energy. This is often represented graphically as having distinct energy levels or electron orbits.
- Spin - Is the intrinsic angular momentum about their own axis of subatomic particles, such as electrons. Electrons also possess orbital angular momentum about the nucleus of the atom. See also Pauli and Spin
- Positron (e+) - the antiparticle of the electron with same mass and spin but with a positive charge equal in magnitude to that of the electron's negative charge. Every particle has a corresponding antiparticle. See also Dirac and the Positron.
- Proton (p) - a subatomic particle with a positive electric charge of equal magnitude, but opposite sign, to the charge on the electron. It is much larger than the electron and has a mass of 1.6726 X 10-27 Kg about 1836 times the electron's mass. Together with the neutrons, the protons form the nucleus of the atom. The number of protons in the nucleus is called the atomic number Z and this determines the atom's chemical properties.
- Neutron (n) - a subatomic particle with no net electric charge. It has a mass of 1.6749 X 10-27 Kg, slightly more than a proton. Free neutrons are not found in nature. They would decay into protons and electrons.
- Fast Neutron - A high energy neutron moving at around 20,000 Km/s with very high energy of more than 1 Mev
- Thermal Neutron - A slow speed neutron moving at about 2.2 km/s with energy less than 1 eV
- Neutrino (ν) - very small subatomic, elementary particles without an electric charge which are produced by the decay of radioactive elements. Neutrinos and anti neutrinos are affected only by the weak nuclear force. Like the neutron, having no charge, they are not affected by electromagnetic forces. See also Pauli's Neutrino
- Antineutrino (-ν) - the antiparticles of neutrinos emitted during beta decay in which a neutron decays into a proton, electron, and antineutrino.
- Nucleus - the very small dense region at the centre of an atom consisting of protons and neutrons. It contains nearly all the mass of the atom.
- Nucleon - the common name for the protons and the neutrons which make up the nucleus of an atom. The mass number A is the number of nucleons in the nucleus.
- Nuclide - a specific type of nucleus associated with a particular atom, characterized by its nuclear properties, such as the number of neutrons and protons and the energy state of its nucleus which differentiates it from the nuclei of other atoms.
- Isotopes - elements or atoms which have two or more nuclides with the same number of protons but a different number of neutrons. They are distinguished by their mass number. That is the total number of protons and neutrons in the nucleus.
- Coulomb Forces - the electrostatic forces between charged particles. Oppositely charged particles attract eachother and similarly charged particles repel eachother. The Coulomb force is the force which binds negatively charged electrons to the positively charged nuclei of atoms. The variation of the force with the distance between particles obeys the inverse square law.
- The Strong Nuclear Force - one of nature's four fundamental forces. It is an attractive force which holds together the nucleons (neutrons as well as protons) in the nucleus of an atom against the repulsive Coulomb forces due to all the positive charges on the protons which would otherwise cause the nucleus to fly apart. The magnitude of the strong nuclear force is much greater than the electrical Coulomb force binding the electrons to the nucleus but it is only effective over a very short range of about 10-15 metres. Though no law governing the action of the strong force between particles has been formulated, some theorists have suggested that, as an approximation, the magnitude varies inversely as the seventh power of the distance between the particles since this is consistent with its very restricted effective range.
- The Weak Nuclear Force - another of nature's fundamental forces, (the others are electromagnetism and gravitation). It governs the the radioactive decay of unstable subatomic particles and nuclear fission. The weak reaction is a form of radioactivity which can transform a neutron into proton and a proton into neutron, resulting in transmutation of the elements, with the following two reactions:
- Electron Decay - A neutron is transformed into a proton by losing a negatively charged electron (beta particle) and an antineutrino and is left with a positive charge.
- Positron Decay - A proton is transformed into a neutron by losing a positively charged positron and a neutrino leaving it with no charge.
n ⇒ p + e- + -νSee also Pauli and Beta Decay.Electron or beta decay is the basis of radiocarbon dating when the radiocarbon isotope of Carbon is transmuted into Nitrogen by beta decay. See explanation ofradiocarbon dating.p ⇒ n + e+ + νIt is positron decay which initiates the Sun's nuclear fusion. A Hydrogen atom (proton) is transformed into a neutron which then fuses with another Hydrogen atom under the Sun's conditions of extreme temperature and pressure to form Deuterium an isotope of Hydrogen. The Deuterium nuclei undergo further fusion reactions to produce Helium with the release of huge amounts of energy. See more about Deuterium Fusion Fuels below. - Binding Energy is derived from the strong nuclear force. It is defined as the energy that is released when a nucleus is assembled from its constituent nucleons and is thus a measure of the amount of energy held within the bonds of the atom and corresponds to the energy required to be put in again to pull the nucleus apart. The mass of the nucleus of an atom is less than the sum of its constituent protons and neutrons (the nucleons) and the difference represents the mass equivalent of the binding energy given by Einstein's mass-energy equivalence formula
Eb=ΔMc2 Where Eb is the binding energy and ΔM is the mass difference. - Mass Defect - Mass Deficiency This is the difference between the mass of a nuclide and the sum of the masses of its individual nucleons. Both of these factors can be measured by mass spectrography and in practice, this provides a way of determining the binding energy. Once this mass difference, is known, the binding energy of any nucleus can be calculated from Einstein's formula E = mc² . The mass defect of a nucleus is thus the mass equivalent of the energy released by the fusion of its constituent nucleons.
Since different isotopes of an element all have the same number of protons and electrons, they have the same or similar chemical properties, but they differ in mass due to the different numbers of neutrons in the nucleus and because of this the nucleus may be unstable. Too many or too few neutrons may make the nucleus liable to decay.
The larger the nucleus, the greater the internal repulsive forces due to the greater number of protons and less energy must be applied to remove a nucleon from the nucleus, hence the binding energy is lower. The greater the binding energy, the more stable the atom is. The following graph shows the binding energy of nucleons of different elements. The total binding energy of the nucleus is given by multiplying the nucleon binding energy by the number of nucleons in the nucleus (The atomic mass A).

When there is an increase in the total binding energy of a system, the system has become more stable by releasing an amount of energy equal to the increase in the total binding energy of the system. Therefore, the energy liberated in the fission or fusion processes is equal to the increase in the total binding energy of the system.
Decay, Fission and Fusion
Three different types of nuclear reactions are possible.
- Nuclear decay occurs when small bits of the atomic nucleus are ejected from an unstable atom transforming it into a different, more stable species. Decay resulting from ejection of Helium atoms (alpha particles) is known as alpha decay, while decay resulting from the ejection of electrons (beta particles) is known as beta decay.
- Fission is the splitting of an atom to form two complete smaller atoms.
- Fusion is the fusing of two smaller atoms to form a larger atom.
The timescales for the decay of unstable isotopes range from fractions of a second to billions of years. See examples below.
When a large unstable nuclide splits into two smaller stable nuclides, the combined binding energy holding each of the smaller nuclides together is less than the energy needed to hold the larger nuclide together and the surplus energy is released.
In the case of fusion, the binding energy of the new, larger stable nuclide is less than the combined binding energy of the individual nuclides which formed it and so the surplus is energy released.
In all three transformations, decay, splitting and fusing, the nuclear reaction is accompanied by a tiny reduction in the total mass of the components and the release of energy.
The fission reaction can proceed uncontrollably and needs to be slowed down whereas the fusion reaction takes prodigious mounts of energy to speed it up and get it started. The fusion process is therefore inherently stable with no threat of runaway.
A word about Iron (56Fe26)
Iron is believed to be the tenth most abundant element in the universe and the fourth most abundant in the Earth's crust.
The graph of binding energies above shows that the Iron atom, situated in the middle of the periodic table, has the highest binding energy and hence is the most stable element. It neither splits nor fuses with other atoms in a nuclear reaction. It represents the dividing line between fission and fusion.
The closer an element's mass number is to that of Iron the the more stable it is and the less the likelihood that it will split or fuse with other atoms easily. The further an element is from Iron in the periodic table the less the binding energy holding it together, so that the elements at the extremes of the periodic table are the least stable and the most reactive. The large heavy atoms at the top of the table can achieve more stable equilibria by fission and the small light atoms at the lower end of the table can reach more stable states by fusion.
Attempting to fuse two atoms which are heavier than iron or to split an atom which is lighter than iron will require energy to be expended and will result in daughter elements, with less stable atoms having less binding energy, further way from the maximum stability point, .
In summary - Nuclear fission in an element heavier than Iron produces energy and fission in any element lighter than Iron requires energy. By contrast, nuclear fusion reactions between elements lighter than Iron produces energy and fusion in elements heavier than Iron requires energy.
Nuclear Decay
Nuclear or radioactive decay was first discovered in some naturally occurring minerals containing elements such as Uranium and Radium. It is the spontaneous process which occurs in radioactive materials by which the nuclei of unstable atoms, the parent nuclides, gradually break up and are transformed into more stable isotopes or into atoms of a different type, the daughter nuclides, consequently losing energy by emitting radiation in the form of ionised particles and / or electromagnetic waves.
Example
The most common isotope of Uranium, U-238 decays by alpha decay to form Thorium-234, a radioactive silvery metal, with the emission of one Helium atom (alpha particle). Thus
238U92 ⇒ 234Th90 + 4He2
The daughter nuclide (thorium-234) has 2 fewer protons and neutrons than the parent nuclide (uranium-238)
At the atomic level, nuclear decay is a random process so that it is impossible to predict when a particular atom will decay. However in practical samples which contain a large number of similar atoms, the average decay rate is predictable.
- Radiation and Energy Release
- Alpha ( α) radiation
- Beta ( β) radiation
- Gamma( γ) radiation
- Neutron Radiation
- Decay Energy
- Nuclear Decay
Rate - Half Life
- Activity Rate
- Radiated Power
The nature of the decay and the energy released depend on the specific elements involved and the type of radiation emitted by the decay. Nuclear decay may be accompanied by up to three types of ionising radiation plus non-ionising neutron radiation.
Alpha particles are Helium - 4 nuclei (2 protons and 2 neutrons). They are large, heavy particles with relatively low energy and are readily stopped by a sheet of paper.
Typically an alpha decay of a single Uranium-238 atom releases 4.3MeV (6.9 X10-13 J) of energy. (but the rate is random and not controlled)
Beta particles are electrons. They have high energy but can be stopped by a thin sheet of aluminium.
The energy released in a typical β-decay compared with alpha emission is very low, with the energy of a single electron being in the order of 1eV (1.6 X 10-19 J). Most of this energy is in the form of the kinetic energy of the emitted electron.
Gamma radiation is high frequency electromagnetic radiation (waves), consisting of high energy photons (not particles). Similar to X rays, but carrying much more energy, gamma rays have high penetrating power but can be absorbed by thick lead shielding or several feet of concrete. (X rays come from the electron shells whereas gamma rays come from the nucleus).
Gamma decay may be spontaneous or induced. This is because atoms can absorb photons and re-emit them at many different energies. The consequence is that the energy released by a single gamma radiation event may be in the range from 10,000eV to 10MeV.
Neutron rays consisting of free high energy neutrons are considered to be non-ionising radiation since neutrons carry no charge. They are not the usually result of decay but of nuclear fission or possibly fusion during which neutrons may be ejected. (See below). Spontaneous neutron emission (decay) can occasionally occur however with some isotopes of atoms containing excess neutrons and some very heavy atoms in which a neutron is simply ejected from the nucleus.
Neutron radiation is best absorbed by materials containing hydrogen such as water or plastic. Shielding against neutron radiation may be provided by 0.5 m to 2 m of water depending on the neutron energy.
The initial energy of a neutron is about 1 MeV (1.6 X 10-13 J)
The break up of a single atom with the release of energy is called a decay event. The amount of energy released in each event is equal to the difference in the binding energy of the atom before the reaction and the sum of the binding energies of the decay products resulting from the reaction. This amount is very small, and is measured in electronVolts (eV) (or Millions of electronVolts MeV)
For a material sample, the number of events occurring is proportional to the number of atoms present, in other words the mass of the sample. For practical samples, it is more convenient measure the rate of energy release rather the absolute amount of energy released, in other words, the power, measured in Watts.
The unit of radioactive decay or the frequency of nuclear events, is known as the Becquerel (Bq) which is defined as one transformation (or decay event) per second.
Since radioactive decay results in the emission of ionised particles, a simple way of measuring the number of events is by counting the number of particles (ions) as they are released using devices like Geiger counters.
The rate of nuclear decay or energy released is thus proportional to the amount of radioactive material remaining in the sample and the frequency of the nuclear events. This is the classic exponential decay represented by the equation:
Nt = N0e-λt
where
N0 is the initial quantity of radioactive (decaying) nuclides measured by mass or numbers of atoms
Nt is the number of nuclides remaining ( not yet decayed) after time t
λ is the decay constant (a measure of the rate at which the nuclide releases radioactive emissions)
e is the natural exponential = 2.718.
Note that nuclear disintegrations are randomly occurring events and the value of λ is a statistical probability.
The rate of radioactive emissions of a radioactive sample is directly proportional to the amount of radioactive nuclides present in the sample. Thus:
A=λNt
so that
A=λN0e-λt
where A is the activity or frequency of nuclear events or disintegrations per second in Becquerels
The mean, or average lifetime of a nuclide, (Τ) also called the time constant , is the sum of the lifetimes of all the individual nuclides in a sample, divided by the total number of nuclides present. It is the reciprocal of the decay constant. Thus:
Mean Life Τ=1/λ
The rate of decay of a radioactive nuclide is usually quoted as its half-life.
The half-life (T½) is the time when the expected number of nuclides that have decayed is equal to half of the original number. (That is the time when when Nt = N0/2)
Substituting in the decay equation (Nt = N0e-λt)
2 = e-λT½
Rearranging, the half life is given by:
T½ = Ln (2) /λ = 0.693 /λ
where Ln (2) is the natural logarithm of 2 = 0.693
Examples 1: Half life
Different materials have different rates of decay and are characterised by their half lives.
The half life of Uranium-238 is 4.5 billion years, roughly the age of the earth. The rarer Uranium-235 (0.72% of all Uranium) has a half life of 704 million years and Plutonium-239 which is virtually non existent in nature has a half life of 24 thousand years. Radium which is found in very small quantities in Uranium ores has thirteen isotopes, the most common of which is Radium-226 which has a half life of 1622 years.
The shorter the half life of a radioactive element, the less of it will remain in the earth's crust.
Cobalt-60 used in medical applications is not found in nature due to its short half life of 5.27 years. It is produced artificially by neutron activation of Cobalt-59
The half life of the Carbon isotope 14C6 decaying to Nitrogen 14N7, as used for radiocarbon dating, is 5,730 years.
The beta decay of the Nitrogen isotope 16N7 transforms the Nitrogen atom into an Oxygen 16O8 atom within a few seconds of the Nitrogen isotope being created.
The activity rate in Becquerels per gram is given by
A/M=λNt / M = N0*ln(2)/T½, x M
where
M is the molar mass of the atom (Assuming the initial quantity of radioactive material = 1 mole)
N0 is the number of atoms per mole (Avogadro's number).
T½ is the half life of the atom in seconds (1 year = 31.536 x 106 seconds)
Example 2 - Cobalt-60 Activity rate
The molar mass M of the Cobalt-60 nuclide is is 59.93 grams
The half life T½ is 5.27 years
Thus the activity rate per gram is
A/M = 6.022 x 1023 x 0.693/ (5.27 x 31.536 x 106) x 59.93 = 41.9 x 1012 Bq/g (or 41.9 TBq/g)
The radiated power associated with each decay event is given by
W = Δm * A/M
or W = E * A/M
Where
W is the radiated power
Δm is the mass defect due to the disintegration of the isotope
E is the binding energy released (equivalent to the mass defect)
Example 3 - Cobalt-60 Radiated Power
Cobalt-60 decays into Ni60.
The mass difference, Δm is 0.00303 amu (atomic mass units).
The corresponding radiated energy E is 2.8 MeV or 4.48 x 10-13 Joules (or Watt seconds)
From the radiated power equation the radiated power per gram of Cobalt-60 is given by:
W = E x A/M = 4.48 x 10-13 X 41.9 x 1012 = 18.77 W/g
Nuclear batteries use the heat evolved from nuclear decay.
Nuclear Fission
Nuclear fission occurs when a neutron collides with a nucleus of a large atom such as Uranium and is absorbed into it causing the nucleus to become unstable and thus split into two smaller more stable atoms with the release of more neutrons and a considerable amount of energy. Nuclear fission can occur naturally with the spontaneous decay of radioactive material or it can be initiated by bombarding the fuel consisting of fissionable atoms with neutrons. Neutrons, which are electrically neutral, can penetrate relatively unhampered into the atomic nucleus and are used as the bullets to initiate the fission rather than protons because, with a positive charge, the protons would be strongly repelled by the positively charged nucleus. See Coulomb Barrierbelow.
- Fissile Material
- Fertile Material
- Fissionable Material
- Fission Energy Release
- Chain Reactions
- Sub-criticality (K < 1): The system can not sustain a chain reaction. A reaction may be started by external neutrons but it dies out fairly rapidly.
- Criticality (K = 1): Every fission cause on average one more and the reaction continues at steady rate. This is the operating state of a power reactor.
- Super-criticality (K > 1): With a very high concentration of fissile material, each fission cause K more fission and the number of neutrons escalates exponentially in an uncontrollable chain reaction a possible explosive release of energy.
- Critical Mass
- Other Nuclear Fission Reactions
- Plutonium
- Production
- Fission
Power from Nuclear Fission
Fissile materials are those fissionable materials which are capable of sustaining a chain reaction when struck by neutrons with low kinetic energy (Slow or thermal neutrons).
The three most important fissile materials which can be obtained in large enough useful quantities are Uranium-233 and Uranium-235, both dense soft silvery metals and Plutonium-239, also a dense silvery white metal.
Fertile materials are isotopes which are capable of becoming fissile by capturing fast moving neutrons possibly followed by radioactive decay. Examples are Uranium-238, Plutonium-240 and Thorium-232.
Fissionable materials are those whose atoms can undergo induced nuclear fission when struck by a free neutron. Fertile materials need a fast moving neutron to initiate fission while fissile materials need a slow moving neutron for fission.
Fissionable materials are not necessarily fissile. Thus, although Uranium-238 is fissionable, it is fertile but not fissile.
Several isotopes of uranium can undergo induced fission. But the only naturally occurring isotope in which fission can be induced with thermal neutrons is Uranium-235 which splits into Barium-141, a soft silvery metal, and Krypton-92, an inert gas, and surplus free neutrons averaging about 2.4 neutrons per event. The following diagram shows the process in more detail.
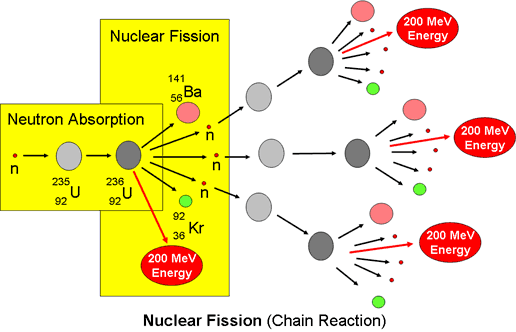
The process can be represented by the following equation:
235U92 + 1 n0 ⇒ 140Ba56 + 96Kr36 + 3 1 n0 + @ 202MeV
The energy released by fission of one atom of Uranium-235 is 200 MeV
The energy released at the atomic level can be calculated from the binding energies of the parent and daughter atoms as shown in the following table:
Binding Energy Change with Fission | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
The Uranium-235 nuclide has a binding energy of about 1786 MeV. The total binding energy of the nuclides of Barium and Krypton which remain after fission amount to about 1952 MeV. The difference of 166 MeV corresponds to the energy released in the fission process. In addition there will also be several small energy releases totaling about 33 MeV associated with ejection of the neutrons and other particles as well as beta and gamma radiation.
Thus the total energy released by the fission of 1 atom of Uranium-235 is about 200 MeV which corresponds to 3.2 X 10-11 Joules.
1 Atom of Uranium-235 weighs 235 amu (atomic mass units) = 3.92 X 10-25 Kg
Therefore 1 kilogram of Uranium 235 contains 1/235amu atoms = 2.54 X 1024 atoms
The energy released from 1 kilogram of fuel is therefore (3.2 X 10-11) X (2.54 X 1024) = 8.1 X 1013 Joules (or 22.5 GWh)
The mass consumed in the transformation is given by Einstein's formula E=mc2
From one atom of Uranium-235 the mass of Uranium actually converted into the 200 MeV of energy is 3.56 X 10 -28 Kg, an almost infinitesimal amount.
From the one kilogram of Uranium-235 fuel consumed the mass of Uranium actually converted into energy is (3.56 X 10 -28) X ( 2.54 X 1024) = 9.04 X 10 -4Kg = 0.9 grams
By comparison, the fission of one atom of uranium produces 10 million times the energy produced by the combustion of one atom of carbon from coal.
Just 0.6 grams of Uranium were consumed by the atomic bomb which devastated Hiroshima in 1945.
As noted above, nuclear fission is initiated by bombarding the nuclei of large unstable atoms with neutrons which cause nuclei to split releasing more neutrons. These neutrons released by the fission process can go on to split further atoms thus releasing even more neutrons. If the number of fissile atoms is small, as in a low mass sample, or if they are widely dispersed, as in an impure sample, most of the neutrons released by the initial fission will not encounter more fissile atoms. They will lose their energy by collision with other atoms and molecules and the reaction will die out. If however there is a large mass of more concentrated fuel, a larger number of neutrons will impinge on more fissile atoms triggering yet more fissions in a chain of events creating more neutrons as each neutron is absorbed. The reaction thus becomes self sustaining and the mass at which the chain reaction just becomes possible is called the critical mass.
K is the effective multiplication factor K is defined as the the ratio of the number of neutrons produced by fission in one generation to the number in the preceding generation. It refers to the conditions of the population of neutrons within the reactor core. This is not the same as the average number of neutrons created by the fission reaction (2.4 in the case of Uranium-235) since some neutrons are absorbed in non-fission reactions and others escape from the system without being absorbed. The way in which a fission chain reaction proceeds depends on the value of K.
Once a critical mass of fuel has been assembled in the reactor core, the population and energy of the neutrons must be controlled to prevent the possibility of a runaway action with disastrous consequences, while at the same time maintaining the chain reaction. This is the function of the control rods whose purpose is to remove neutrons from the reactor core.
In practical terms the effective critical mass depends on many other attributes, such as the degree of enrichment of the fuel, its shape, temperature, density, and whether it is contained within a neutron-reflective substance. The minimum critical mass of Uranium-235 is a 52 Kg sphere 17 cm in diameter. For Plutonium-239 the corresponding figures are 10 Kg in a sphere of 9.9cm diameter. Taking into account the degree of dilution of the desired isotope in the fuel bulk, the critical mass of Uranium-235 enriched to 20% will be 400Kg rising exponentially as the enrichment is decreased further.
The content of fissile Uranium-235 used in nuclear reactors is less than 1% in reactors using natural, unenriched fuel and up to 5% or 6% in reactors using enriched fuel. Almost all of the balance (94% to 99%) is non fissile Uranium-238 with a tiny amount (0.0055%) of Uranium-234. The free neutrons bouncing around inside a Uranium reactor don't just react with the Uranium-235, they can also react with other Uranium isotopes they encounter as well as any other elements present in the nuclear pile to produce other transmutations and disintegrations.
On a cosmic timescale, Plutonium has a relatively short half life of only 24,000 years which means that it does not occur naturally on earth. On a contemporary timescale however 24,000 years is an awful long time for Plutonium waste, produced by nuclear reactions, to remain radioactive.
Since the bulk of the fuel charge in a conventional Uranium fuelled reactor is Uranium-238, the free neutrons released by the fission of Uranium-235 have a high probability of colliding with Uranium-238 atoms. The collision of both fast or slow neutrons with Uranium-238 can result in the capture of the neutron and the transformation of the Uranium-238 into Uranium-239, an unstable isotope which decays within a few hours to become Neptunium-239 with the emission of a beta particle (an electron). This unstable Neptunium isotope undergoes further beta decay losing another electron during the next several days to become the more stable Plutonium-239, one of fifteen isotopes of Plutonium, which has a half life of 24,000 years.
Plutonium-239 however is fissile, more readily so than Uranium-235 emitting more excess neutrons per fission than Uranium-235 so that a chain reaction can be achieved with less than one third of the critical mass of fuel . Plutonium-239 can however also capture fast neutrons as well as slow neutrons causing even more fissions.
A slow neutron can split Plutonium as follows into Barium and Strontium with the emission of 3 neutrons and energy of 207 MeV which is not much different from the energy released by the fission of Uranium-235
239Pu94 + 1 n0 ⇒ 142Ba56 + 95Sr386 + 3 1 n0 + @ 207 MeV
The production of Plutonium-239, the fuel used in nuclear weapons, is thus inevitable in conventional Uranium fuelled nuclear reactors and Plutonium fissions provide about one-third of the total energy produced in a typical mature Uranium fuelled commercial nuclear power plant.
Nuclear power generation is now firmly established throughout the world and is based on controlled nuclear fission. See the page on Nuclear Fission - The Practice
Nuclear Fusion
The Holy Grail of nuclear energy, nuclear fusion is the process by which the Sun generates its prodigious energy providing us with the warmth and light we receive. It is the process by which the nuclei two light atoms combine to form a single, bigger nucleus of a new atom releasing large amounts of energy as a consequence. In 1939 Hans Bethe explained that this process occurs in the stars all over the universe but up to now we have not been able to successfully duplicate this process on earth despite over 70 years of trying, but at last we are coming close.
The great attractions of nuclear fusion as an energy source are that the fuel, mostly isotopes of hydrogen, is plentiful and easy to obtain, and the elements produced as a result of the fusion are usually light and stable atoms rather than the heavy radioactive products which result from nuclear fission. Furthermore, the potential release of energy per unit mass of the fuel is much higher in the case of fusion than in fission since reactions allowing greater increases in binding energy are possible with fusion reactions. See the graph of binding energy above.
- The Coulomb Barrier
- A Note About Temperatures:
Unfortunately, although bringing about fusion is theoretically possible, achieving it in practice is fraught with major difficulties.
For fusion between two positively charged nuclei to take place, they must get close enough to eachother to undergo a nuclear reaction. For this to occur the nuclei must overcome the energy barrier due to the "repulsive" electrostaticCoulomb force, known as the Coulomb barrier, between the nuclei, to force them close enough to each other to come within the influence of, and be captured by, the "attractive" Strong nuclear force which holds the nucleons in each nucleus together.
| 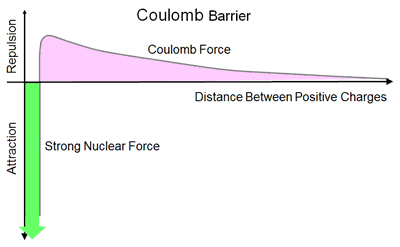 |
The magnitude of the Coulomb barrier corresponds to the work done or energy needed for the two nuclei overcome the Coulomb force to come together. This is equal to the potential energy between the particles and is given by the Coulomb force between two nuclei multiplied by the distance between them integrated over the distance over which the force is effective. Thus for fusion to occur, the nuclei must have at least enough kinetic energy to exceed the Coulomb repulsion and this is proportional to the mass of the nuclei and the square of their velocity. The magnitude of the this kinetic energy is also directly proportional to the temperature of the nuclei.
All fusion reactions require the fusing nuclei to have extremely high kinetic energies in order to overcome the Coulomb barrier and get close enough to each other to fuse. Their temperature must therefore be extremely high, 100 Million°C or more, so that the nuclei collide with eachother at great speed.
The temperature and energy of a moving gas or plasma particle are directly proportional to its velocity squared.
In nuclear physics, temperature is typically used to express the "average" energy of the particles in a fuel sample. Conversely, it is also common to express very high temperatures in terms of energy units (Joules or electronVolts).
The energy in Joules is given by multiplying the temperature in degrees Kelvin by Boltzmann's constant of 1.38 X 10-23 Joules/degree Kelvin (J/K)
Thus:
1 Joule (J) ≡ 6.24 x 1012 Million electronVolts (MeV)
and
1000 electronVolts (KeV) ≡1.16 X 107 °K
Fusion temperatures are so high that °Kelvin and °Celsius are almost the same and are often used interchangeably
As a point of reference, the temperature at the core of the Sun is around 1.5 X 107 °K. This does not mean that all the atomic particles are at that temperature. This temperature represents the average energy of the atomic particles in the core. In thermal equilibrium, the atoms have a range of velocities (energies) described by theMaxwell-Boltzmann distribution which represents the number of particles at each energy level in the sample.
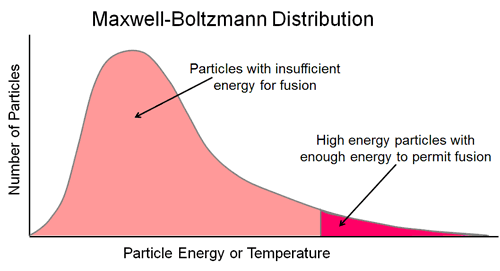 |
The energy distribution shows a very high percentage of particles with energy levels around the average level and relatively low percentage of very low and very high energy particles at the lower and upper tails of the distribution. In absolute terms however, because of the extremely high particle density at the centre of the Sun, there will be a very large number of particles in the longer, upper tail with energies or temperatures many times higher than the mean
|
- The calculation of the Coulomb barrier determines the "average" energy per proton needed for fusion, however these energies are distributed according to the bell shaped Maxwell-Boltzmann distribution. Within its long tail there is a sufficiently large number of particles whose energy is much larger than the average and thus enough to initiate fusion.
- It is not necessary for the incident protons to have sufficient energy to overcome the Coulomb barrier entirely. Due to the wave-particle duality and the statistical probabilities of the properties of very small particles, as explained by quantum mechanics, the protons can also pass through the barrier by a phenomenon known asquantum tunnelling, provided the barrier height is not too high above the kinetic energy of the incoming particle.
- Fusion Fuels
- Deuterium
- Tritium
- Fusion Energy Release
- The first reaction produces the He3 isotope of Helium and an energetic neutron.
- The second reaction produces Hydrogen and Tritium which is mildly radioactive.
- The third reaction is the well known D-T reaction between Deuterium and Tritium which produces a Helium atom (alpha particle) and a high energy neutron.
- The fourth reaction between Deuterium and the Helium isotope He3 produces a Helium nucleus (alpha particle) and 2 energetic neutrons and is known as the D-He3 reaction.
The Coulomb barrier between two protons in free space is 3.43 MeV and this corresponds to a temperature of 4 × 1010 °K. This is over 1000 times the temperature of 1.3 KeV (1.5 X 107 °K) at the core of the Sun. How then can proton fusion occur on the Sun with such low particle energy levels? There are two explanations for this apparent anomaly.
Fuel Density
For significant fusion to take place, the particle density of the fuel must also be very high to provide sufficient opportunities for collisions between the particles to occur.
In a star, the high temperatures are provided by the self sustaining nature of the nuclear reaction itself and the density of the fuel is maintained by the star's massive gravity. On earth, attempts have been made to create these extreme conditions in a plasma of ionised gases but containment is a serious problem since no known materials for containing the fuels can withstand such high temperatures. An alternative is to capture the energy from a series of tiny, controlled thermonuclear explosions which has so far been more successful. See Nuclear Fusion - The Practice
Fusing two light nuclei can liberate more than the fission of Uranium-235 or Plutonium-239. Ideal fuels are the lightest elements since they experience the lowest repulsive force, or Coulomb barrier, between their nuclei, so that the energy needed to bring about fusion is reduced. The most common fuels used in fusion attempts are Deuterium and Tritium, gaseous isotopes of hydrogen, in the so called D-T reaction.
Deuterium (2H) also known as heavy Hydrogen is a naturally occurring, stable isotope of Hydrogen found in the earth's oceans where it accounts for approximately 0.015% of the Hydrogen atoms, or 0.030% of the weight of Hydrogen. Deuterium is extracted from the water by a variety of separation methods which exploit the small differences in physical and chemical properties between Deuterium and Hydrogen.
The Deuterium in one gallon of sea water has the energy content of 300 gallons of gasoline and the oceans contain enough energy to supply the world's energy needs for thousands of years..
Tritium (3H) is also an isotope of Hydrogen. It is radioactive with a half-life of 12.3 years decaying at the rate of 5.5% per year by beta decay into Helium-3 with the release of an electron and of 18.6 keV of energy. Because of its short half-life, only tiny quantities are found naturally as the result of the reactions of cosmic rays with atmospheric gases. Supplies of Tritium are produced as a by-product of other nuclear reactions, notably those involving Lithium and much of it is reserved for nuclear weapons. It is consequently both rare and very expensive.
Due to their low energy, the emitted electrons can not penetrate the skin and so Tritium is not considered an external radiation hazard though it could cause damage if inhaled or ingested. Tritium is however often used as a biological tracer in medical research because of its short half-life and low radiation.
The fusion of Hydrogen nuclei (protons) in a proton-proton reaction is not practical since it would require too much energy or too high a temperature to start. The fusion of Deuterium with Tritium needs a temperature of 100 million to 150 million degrees Centigrade. All other fusion reactions need even higher temperatures.
The D-T fusion reaction between Deuterium and Tritium is shown below:
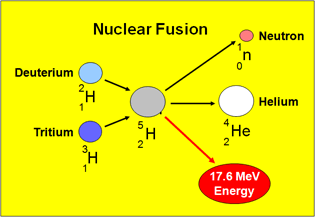
2H1 + 3H1 ⇒ 4He2 + 1 n0 + @ 17.6 MeV
The diagram above shows that the fusion of one atom of Deuterium with one atom of Tritium to form one atom of Helium (also called an alpha particle) releases about 17.6 MeV of energy.
Note that this reaction results in the release of a troublesome surplus neutron which can cause problems in practical fusion reactors, such as the Tokamak. Since they carry no electric charge, neutrons are not constrained in the the plasma by the magnetic field and can migrate to the walls of the reactor where they can react with parts of the reactor construction materials which may consequently become radioactive.
This unavoidable side effect can however be turned to an advantage. By using a Lithium metal blanket to capture the surplus neutrons in the reactor, two useful fission reactions are possible, both of which result in the release of alpha particles (Helium nuclei) and, more importantly, the production of Tritium, the scarce, radioactive, isotope of Hydrogen, which is one of the basic fuels for the fusion reaction.
The first reaction is exothermic and uses the isotope Lithium-6 to capture slow neutrons producing Tritium while releasing an alpha particle and 4.8 MeV of energy as follows:
6Li3 + 1 n0 ⇒ 4He2 + 3T1+ @ 4.8 MeV
The second reaction is endothermic using the isotope Lithium-7 to capture fast neutrons, also producing Tritium and releasing an alpha particle while leaving a free neutron and absorbing 2.5 MeV of energy.
7Li3 + 1 n0 ⇒ 4He2 + 3T1+1 n0 - @ 2.5 MeV
Natural Lithium is relatively abundant in the earth's crust and is typically composed of 92.6% of the isotope Lithium-7 and 6.4% of Lithium-6.
The energy released at the atomic level by the fusion of Deuterium and Tritium can be calculated from the binding energies of the parent and daughter atoms as shown in the following table:
Binding Energy Change with Fusion | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
The table shows that the combined binding energy of the Deuterium and Tritium atoms of 10.7 MeV increases to 28.3 MeV when the atoms fuse into Helium releasing energy of 17.6 MeV, equivalent to 2.8 X 10-12 Joules.
Note that 80% of the released energy is carried by the neutron with the Helium alpha particle accounting for only 20%
The atomic mass of the Deuterium nuclide is 2 amu = 3.34 X 10-27Kg
1 Kg of Deuterium therefore contains 1Kg/2 amu = 2.99 X 1026 atoms.
The atomic mass of the Tritium nuclide is 3 amu and hence 1.5 Kg of Tritium also contains 2.99 X 1026 atoms.
The energy released by fusion of 1 atom of Deuterium with 1 atom of Tritium is 17.6 Mev = 2.82 X 10-12 Joules.
The energy liberated by the fusion of 1 Kg of Deuterium with 1.5 Kg of Tritium is therefore 2.82 X 10-12 X 2.99 X 1026 = 8.43 X 1014 Joules = (8.43 X 1014) / (3.6 X 1012) GWHours = 234 GWHours.
This energy appears in the form of heat. If it was used to generate electricity in a conventional steam turbine power plant with an efficiency of 38%, it would provide 88,900 MWH of electricity which is near enough equivalent to one year's operation with a constant output power of 10 MWatts.
Fission - Fusion Energy Comparison
Note that the 234 GWH (8.43 X 1014 Joules) released by the fusion of 2.5 Kg of the fuel in the D-T (40-60 proportion) reaction above is equivalent to 93.6 GWH (3.37 X 1014Joules) per Kg. This is over four times the 22.5 GWHours (8.1 X 1013 Joules) of energy released by the fission of 1 Kg of Uranium - 235 (see fission energy release above). The fusion reaction also uses safer fuels which are inexpensive, more plentiful, and easier to manage, leaving behind much more benign waste products resulting from the process.
To put these values into perspective, 1 Kg of petrol (gasoline) has an energy content of about 4.7 X 107 Joules or (4.7 X 107) / (3.6 X 106) kWH = 13 kWH, less than one millionth of the energy from either nuclear fission or fusion. See also Energy Content Comparison Table
There are actually four possible fusion reactions which could take place in a reactor fuelled by Deuterium only.
2H1 + 2H1 ⇒ 3He2 + 1 n0 + @ 3.3 MeV
2H1 + 2H1 ⇒ 3H1 + 1 H1 + @ 4.0 MeV
2H1 + 3H1 ⇒ 4He2 + 1 n0 + @ 17.6 MeV
2H1 + 3He2 ⇒ 4He2 + 1 H1 + @ 18.3 MeV
The results of these four reactions can be summarised as follows:
62H1 ⇒ 21H1 + 24He2 + 21 n0 + @ 43.2 MeV
The first two of the above reactions using only Deuterium as the fuel, known as D-D reactions are equally probable. This fuel combination has the advantage that the Deuterium fuel does not have the mildly radioactive properties of Tritium, but the energy release is relatively low.
The third and fourth reactions involve the Deuterium fuel reacting with the fusion products of the first two reactions producing a much higher energy release.
Other promising fusion fuel candidates are the Hydrogen-1 (proton) with / Boron reaction, which releases Helium (alpha particles).
Solar fusion is initiated by the fusion of two Hydrogen nuclei (protons) in the following reaction in which the two protons fuse to form a Deuterium atom as one proton is transformed into a neutron, with the release of a positron and a neutrino and 0.42 MeV of energy.
1H1 + 1H1 ⇒ 2H1 + e+ + ν @ 0.42 MeV
At the same time, the positron emitted by the beta decay of the proton is almost immediately annihilated with an electron, and their combined mass energy, as well as their kinetic energy, is carried off by two gamma ray photons.
e+ + e- ⇒ 2 γ @1.02 MeV
Proton - Proton (p-p) Chain Reaction
After the initial reaction, solar fusion continues with more protons reacting with the Deuterium produced in the first reaction to form a light isotope of Helium releasing more energy in the following reaction and initiating a chain of reactions.
2H1 + 1H1 ⇒ 3He2 + ν @ 5.49 MeV
In turn, the products of this second reaction fuse with even more particles to form ever more complex, heavier nuclei releasing more energy in further reactions such as theDeuterium D - D reactions above.
Power from Nuclear Fusion
Unfortunately immense amounts of energy are needed to create the conditions for self-sustaining fusion to take place and in practice there are serious technical problems to overcome in order to achieve a net energy gain. These are considered in the section on Nuclear Energy - The Practice
Physiological Effects of Nuclear Radiation
The term Nuclear Radiation normally refers to radiation with sufficient energy to cause ionisation of the materials on which it impinges.
- The Cause
- The Exposure
- The Effect
- 1 Joule of beta or gamma radiation absorbed per Kg of tissue (1 Gy) has 1 Sievert (Sv) of biological effect. (Q=1)
- 1 J/Kg (1 Gy) of alpha radiation has 20 Sv effect (Q=20)
- 1 J/Kg (1 Gy) of neutrons has a 10 Sv effect (Q=10)
- Radiation Dose Safety Limit
- Radiation Dose from Medical Radiological Tests
- Single chest X-ray 0.1 mSv
- Single mammogram 3 mSv
- Full body CAT or CT scan 10 mSv
High-energy alpha (α), beta (β) and gamma (γ) radiation can transfer their energy upon interaction with other matter, knocking out electrons from neutral atoms or molecules on which they may impinge, leaving electron-deficient atoms or molecules called ‘positive ions’ and free electrons in a process known as ionisation. This ionisation can be measured by counting the number of ions formed using devices such as Geiger counters.
Alpha and beta rays are relatively harmless unless emitted inside the human body, but gamma rays cause damage similar to, but more serious than, X rays such as burns and cancerous mutations.
Very high levels of radiation may not just strip electrons from their atoms, if high enough, it can cause disintegration of the atomic nucleus creating radioactive isotopes or in extreme cases causing the nucleus to split into smaller particles (nuclear disintegration or fission).
Neutron radiation can induce non-radioactive atoms, including the body tissues, to become radioactive, which makes it one of the most dangerous radiations. This capability however also has practical applications in manufacturing isotopes for use in nuclear medicine.
See more about RF radiation damage and X-rays.
The exposure to ionising radiation is measured in terms of the amount of energy imparted by the radiation to the material through which it is passing and is known as the radiation dose, expressed in ‘Gray’ (Gy) after the British medical physicist. One Gray corresponds to the deposition of 1 Joule of energy in 1 kg of the exposed material.
When nuclear radiation strikes complex biological molecules, such as proteins or nucleic acids, it may fracture the molecules and prevent their proper functioning. This can result in rupturing the cell membranes, loss of cell vitality, decreased enzyme activity, initiation of cancer, and genetic mutations. Rupturing of the cells causes them to lose their contents and die and ultimately the functions associated with the cells cease. Death occurs because of the direct loss of vital organs or because of secondary infections resulting from the breakdown of the immune system. The effects are cumulative and depend on the type of radiation and the dose received.
The magnitude of the effect depends on the intensity of the radiation, the distance from the source, the presence of absorbent materials in the intervening space (including air) and the duration of the exposure. Radiation workers wear badges made of photographic film which indicate the exposure to radiation.
The actual biological damage caused by the radiation depends on its physiological effect and is measured in Sieverts (Sv) after the Swedish medical physicist. Sieverts are related to Gray by a so called "quality factor" (Q) representing their potential for doing damage. A more appropriate name for the weighting factor would perhaps have been the "damage factor".
The Gray is the dose, the Sievert is the corresponding biological risk.
The Sievert is a relatively large unit with an exposure of 1 Sievert increasing the risk of cancer by 5%, while an exposure of 10 Sieverts can be fatal within days. Typical exposure levels are much lower and are measured in millisieverts (mSv).
The average exposure in the United States, from natural sources of radiation, mostly cosmic radiation and radon gas released from the earth's crust, is 3 mSv (300 millirems) per year at sea level and slightly higher at higher elevations. Similarly the average yearly background radiation in the UK is 2 mSv.
The current federal occupational limit of exposure per year for an adult worker "as low as reasonably achievable (ALARA); however, not to exceed 20 mSv (2,000 millirems)above the 3 mSv of natural sources of radiation and any medical radiation. For workers using radiation the limit is slightly higher at 50 mSV above background . Readings typically are taken monthly. A federal advisory committee recommends that the lifetime exposure be limited to a person's age multiplied by 10 mSv (example: for a 65-year-old person, 650 mSv).
Typical exposures from medical investigations using X-rays are as follows:
See also Radioisotope Thermoelectric Generators (RTG) - The Nuclear Battery
See Nuclear Energy - The Practice for electricity generation by nuclear fission and nuclear fusion.
Return to Electrical Energy Supply Overview
No comments:
Post a Comment